(An excerpt from the Mectizan Donation Program Annual Highlights for 2021)
Mectizan is donated free of charge to eligible countries where river blindness and/or lymphatic filariasis are endemic.
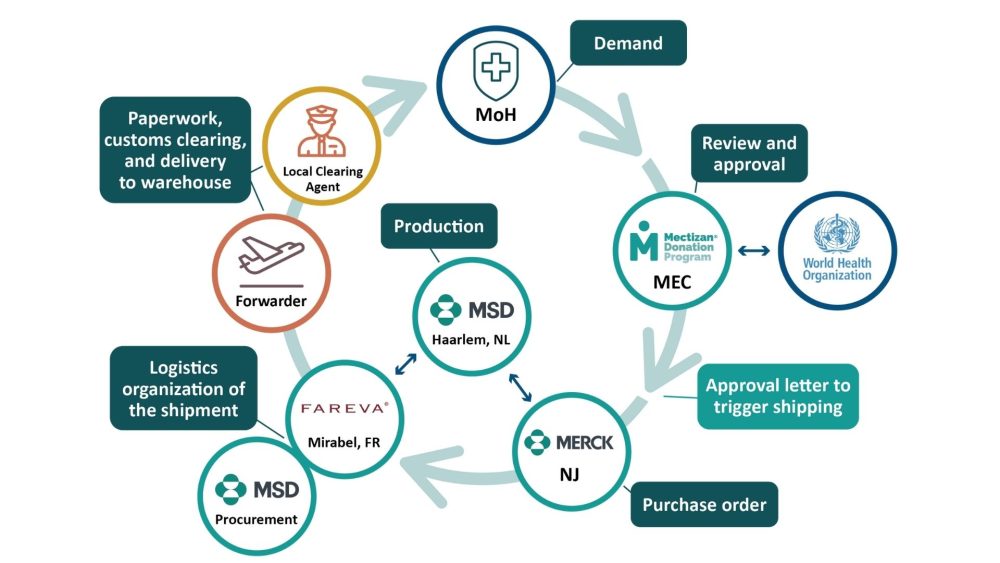
The application is just the first of many steps to getting treatments to people who need them. The supply chain for delivering Mectizan to endemic countries is highly complex, involving many stakeholders.
The Mectizan journey begins with the Ministry of Health’s submission of the Joint Request for Selected Preventive Chemotherapy Medicines (JRSM) to both WHO and MDP. Upon review and approval by the Mectizan Expert Committee (for initial applications) or MDP (for re-applications), Merck & Co., Inc. is notified to initiate shipment. Mectizan tablets are manufactured by MSD* in the Netherlands. Working in partnership with Fareva in France, MSD then packages and ships the medicine to the national drug warehouse in the beneficiary country. The donation agreement stipulates that Mectizan must be imported free of customs duty and fees; in 2021 unexpected clearance fees caused delays in some countries.
From there, the country program takes over and the medicine is transferred down to the district or sub-district level. At this point, health workers must transport allotments to each village, no matter how remote. A process that begins as an email often ends on a bicycle or boat!
In addition to the typical logjams in any supply chain, throughout 2021 our country partners were forced to address issues created by the pandemic—from lack of international transport carriers, to port shutdowns, to slowness of local administration. Some countries were unable to fulfill their 2021 MDA goals. Since the tablets have a 3-year shelf life, many countries saved their shipments for 2022 catch-up campaigns. We look forward to reporting good news about high treatment coverage in 2022.
